Copper Smelting and Refining Process
Refined Copper Smelting and Refining Process
01Formulation of copper concentrate
Large ships bring copper concentrates from overseas mines. The copper concentrates are unloaded by the berth's massive unloader, after which they are transferred to the storage yard where they are sorted and stored. After the concentrates are separated into different strage bins by formulation quality, they are transferred to a flash smelting furnace.



02Flash smelting furnace
The copper concentrates are fed through the flash smelting furnace with oxygen-enriched air. In the furnace, the concentrates are instantly oxidized, after which they melt and separate by their own reaction heat into copper matte with a grade of 65% and slag consisting of iron oxide, silica, and other compounds.
Reaction in the flash smelting furnace
CuFeS2 + SiO2 + O2 → Cu2S・FeS + 2FeO・SiO2 + SO2 + Reaction heat



03Converter furnace
The matte produced by the flash smelting furnace is transferred to the Converter furnace. Oxygen-enriched air is blown into the Converter furnace to oxidize the matte further to create blister copper with a grade of approximately 99%.
Reaction in the converter
Cu2S・FeS + SiO2 + O2 → Cu + 2FeO・SiO2 + SO2 + Reaction heat



04Anode furnace
Butane gas is blown into the anode furnace as a reductant to remove the oxygen in the blister copper, which is refined to a purity of approximately 99.5%.

05Anode casting wheel
The refined blister copper is poured into casting molds lined up side by side on the revolving circular table of a casting wheel and cast into anode plates for electrolytic refining. The anode plates are approximately 1m x 1m x 0.05m in size, and weigh 380 kg per plate.


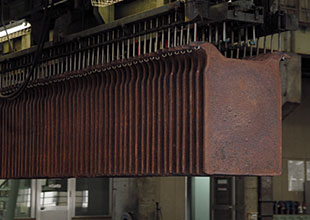
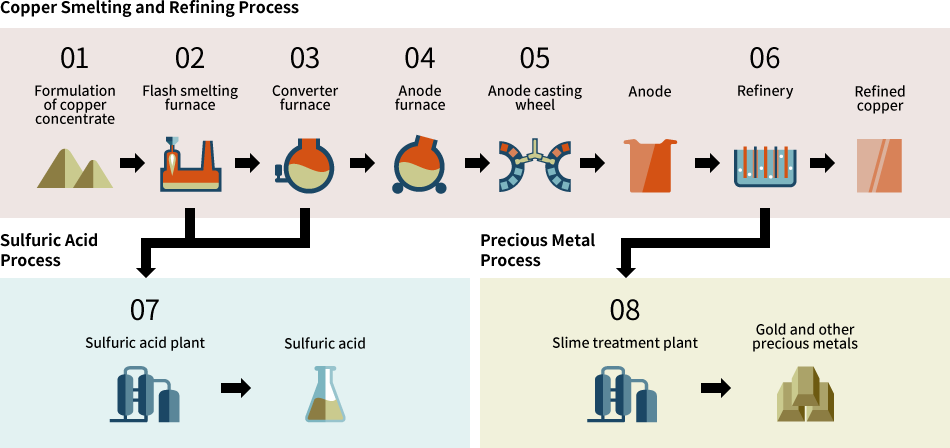
06Refinery
Anode plates and stainless steel cathode plates are alternately set into the electrorefining cell, where a proper level of DC current is supplied. Dissolved copper from the anode is electrolytically deposited on the stainless steel cathode plate. After about 10 days of electrolysis, the cathode is lifted out and stripped from the stainless steel plate, resulting in the completion of refined copper (with grade of 99.99%) as a final product.
The refined copper is shipped in stacked bulks, which are 1m in length and width and approximately 80kg in weight.


Sulfuric Acid Production Process
07Sulfuric acid plant
The gases expelled from the flash smelting furnace and converter furnaceinclude highly concentrated SO2 gas.
The SO2 gas is first recovered from the waste heat boiler. After electrostatic precipitators remove dust particles in the gases, the gas is transferred to the sulfuric acid plant.
At the sulfuric acid plant, the catalytic reaction of vanadium oxides causes SO2 to oxidize into SO3, which is absorbed in water to yield concentrated sulfuric acid.
The manufactured concentrated sulfuric acid and oleum is shipped by ship and lorry.


Gold Refining Process
08Slime treatment plant
Slime generated in the electrolytic refining process contains precious and rare metals such as gold, silver, slenium, tellurium, etc. These precious and rare metals are recovered through processes in the slime treatment plant.
The powdered gold from the slime treatment plant is melted and cast into ingots.



